How to release brass at home. Annealing, hardening and heat treatment of brass. Stress Relief Annealing
Are you interested in annealing, hardening and heat treatment brass? Supplier Evek GmbH offers to buy brass at affordable price in a wide range. We will provide delivery of products to any point of the continent. The price is optimal.
Technology Choice
The types of brass heat treatment are determined by the percentage of zinc in the alloy, as well as the type of state diagram, which type of brass the alloy belongs to - single-phase or two-phase. Supplier Evek GmbH offers to buy brass rolled products of domestic and foreign production at an affordable price in a wide range. We will provide delivery of products to any point of the continent. The price is optimal.
Heat treatment of single-phase (plain) brasses
For such varieties, recrystallization or conventional annealing is used. The goal is to relieve internal stresses that may appear in the process of plastic deformation of the material. The annealing mode depends on the concentration of zinc in the alloy: with an increase in this parameter, the required heat treatment temperature decreases, but not more than to 300 °C. The annealing efficiency depends on the final grain size in the microstructure. They are installed according to the readings of a metal-instrument microscope, or according to the reference structures, which are given in GOST 5362.
Atmosphere for annealing
It is not recommended to perform heat treatment in a normal atmosphere containing a significant amount of oxygen. This leads to an uneven decrease in grain size, and oxide spots are clearly distinguished on the surface of the alloy, which have to be removed by etching the alloy in a solution of orthophosphoric acid or potassium bichromate. More effective method heat treatment is vacuum annealing, or the use of a protective atmosphere inert gases. At the same time, zinc burnout is also reduced.
Heat treatment of two-phase brasses
Multiphase brasses are obtained by adding alloying elements other than zinc - iron, aluminum, lead, etc. Each of the brass grades has its own recrystallization annealing temperature. The most commonly used modes are:
Buy. Supplier, price
Are you interested in annealing, hardening and heat treatment of brass? Supplier Evek GmbH offers to buy brass at the manufacturer's price. We will provide delivery of products to any point of the continent. The price is optimal. We invite you to partner cooperation.
Annealing of steel parts
To facilitate the mechanical or plastic processing of a steel part, its hardness is reduced by annealing. The so-called full annealing consists in the fact that the part or workpiece is heated to a temperature of 900 ° C, kept at this temperature for some time necessary to heat it throughout the volume, and then slowly (usually together with the furnace) cooled to room temperature.
Internal stresses that have arisen in the part during machining are removed by low-temperature annealing, in which the part is heated to a temperature of 500-600°C, and then cooled together with the furnace. To relieve internal stresses and some decrease in the hardness of steel, incomplete annealing is used - heating to 750-760 ° C and subsequent slow (also together with the furnace) cooling.
Annealing is also used in case of unsuccessful hardening or when it is necessary to re-harden the tool for processing another metal (for example, if a copper drill needs to be re-hardened to drill cast iron). During annealing, the part is heated to a temperature slightly below the temperature required for hardening, and then gradually cooled in air. As a result, the hardened part again becomes soft, machinable.
Copper is also subjected to heat treatment. In this case, copper can be made either softer or harder. However, unlike steel, copper is hardened by slow cooling in air, and copper acquires softness by rapid cooling in water. If a copper wire or tube is heated red-hot (600°C) over a fire and then quickly immersed in water, the copper will become very soft. After giving desired shape the product can again be heated on fire to 400 ° C and allowed to cool in air. The wire or tube will then become solid. If it is necessary to bend the tube, it is tightly filled with sand to avoid flattening and cracking.
Annealing brass improves its ductility. After annealing, brass becomes soft, easily bent, knocked out and well drawn. For annealing, it is heated to 600°C and allowed to cool in air at room temperature.
Annealing and hardening of duralumin
Annealing of duralumin is carried out to reduce its hardness. The part or workpiece is heated to approximately 360°C, as in hardening, held for some time, and then cooled in air. The hardness of annealed duralumin is almost half that of hardened duralumin.
Approximately, the heating temperature of a duralumin part can be determined as follows: At a temperature of 350-360 ° C, a wooden torch, which is carried over the hot surface of the part, is charred and leaves a dark mark. Precisely enough, the temperature of a part can be determined using a small piece of copper foil (the size of a match head), which is placed on its surface. At 400°C, a small greenish flame appears above the foil.
Annealed duralumin has a low hardness, it can be stamped and bent in half without fear of cracking.
hardening. Duralumin can be hardened. During hardening, parts made of this metal are heated to 360-400 ° C, held for some time, then immersed in water at room temperature and left there until completely cooled. Immediately after this, duralumin becomes soft and ductile, easy to bend and forge. He acquires increased hardness after three to four days. Its hardness (and at the same time brittleness) increases so much that it cannot withstand bending through a small angle.
Duralumin acquires its highest strength after aging. Aging at room temperature is called natural, and at elevated temperatures- artificial. The strength and hardness of freshly hardened duralumin, left at room temperature, increases over time, reaching the highest level after five to seven days. This process is called the aging of duralumin.
<<<Назад
Soldering or welding aluminum? What is the difference and which is better?
First, let's look at the definitions. Welding is the process of making all-in-one joints by establishing interatomic bonds. Brazing is the process of joining metals in a heated state by melting an alloy, a solder, such as the metals to be joined.
In other words, when welding, the edges of the welded parts are melted and then frozen. When soldering, ordinary metal is heated only to a certain temperature, and the connection is obtained by surface diffusion and chemical reaction of the solder and fused metals.
So which is better, soldering or aluminum welding?
To answer this question, consider the main methods of brazing and welding aluminum alloys, their advantages and disadvantages.
aluminum welding.
The four types of welding most commonly used in aluminum welding are:
1. Electrode welding or TIG welding. As an electrode that does not consume, tungsten is used with special alloying additives (lanthanum, cerium, etc.).
Through this electrode, an electric arc occurs, which melts the metal. The welding wire is manually fed by the weld pool. The whole process is very similar to conventional gas welding, only the heating of the metal is not carried out by burning a torch, but by an electric arc in a protective environment. Such welding is carried out exclusively in an argon or helium environment or mixtures thereof.
Is there a difference between welding argon and helium? There is. The bottom line is that helium provides a more compact combustion arc and therefore deeper and more efficient penetration of base metals. Helium is more expensive and its consumption is much higher than that of argon. In addition, helium is very liquid, which creates additional problems during production, transportation and storage.
Therefore, it is recommended to use it as a shielding gas only when welding large parts, where deep and efficient fusion of the weld edges is required. In practice, helium is rarely used as an inert gas, since almost the same penetration effect can be achieved in argon, which only increases the welding current. TIG welding of aluminium, as a rule, results in alternating current.
Why with alternating current? It's all about aluminum oxide, a small amount of which, when welding, is inevitably present in all types of welding. The fact is that the melting point of aluminum is about 660 degrees. The melting point of aluminum oxide is 2060. Therefore, aluminum oxide cannot melt in the weld - there is not enough temperature.
And there will be no manual for high quality welding oxide. What to do? The income comes from feedback polarity, which has a very interesting feature for cleaning the seam from unnecessary impurities. This property is called cathodic dispersion. However, reverse polarity welding current has a very low melting power. Therefore, the arc also contains current components of straight polarity, which are designed to be insensitive, but melting metals.
And the exchange of direct and reverse polar currents is an alternating current that combines both cleaning and melting properties.
2. Welding with a consumable electrode or semi-automatic welding (MIG welding). All this applies to this type of welding, with the only difference being that, as a rule, the only constant "cleaning" is the replacement of the poles of the arc streams and does not go through the tungsten electrode and directly through the welding wire melted during welding.
Ordinary semi-automatic machine is used for welding, but with higher requirements for wire feeding. This type of welding is characterized by high productivity.
Manual arc welding with coated electrodes (MMA welding). It is used for welding hard parts with a thickness of 4mm or more. It is applied to reverse polarity flow and has a low quality seam.
4. Gas welding of aluminum. It can only be used for a limited number of aluminum alloys, which are characterized by disgusting weld quality. It is very difficult and not accessible to every mortal.
In practice, this is almost never used.
Leaving exotic welding (friction welding, explosion welding and plasma) alone, the quality of the welded joint and the prevalence far outstrip the form, AC argon arc welding.
It allows you to weld pure aluminum, duralumin, silin, etc., alloys from a few millimeters to several centimeters. In addition, it is the most economical and the only one possible for nuclear welding and some other aluminum alloys.
Aluminum soldering
Usually separate low temperature (soft soldering) and high temperature (soldering soldering), the type of soldering.
Soldering aluminum soft solder is usually done with a conventional soldering iron and can be used as a specialty solder for high zinc aluminum and conventional lead-tin solder. The main problem with this type of soldering is the fight against light aluminum oxide. To neutralize it, it is necessary to use various types of fluxes, soldering fats and special types of soldering. In some cases, the surface of the aluminum is electroplated with a thin layer of copper that is already soldered by traditional soldering.
However, the use of electroplated coatings is far from technologically feasible and economically feasible. In any case, soldering aluminum alloys at low temperatures is quite difficult, and the quality of solder joints is usually more than average. In addition, due to the heterogeneity of metals, the bonded joint is susceptible to corrosion and must always be varnished or painted. Soldering with soft sockets cannot be used for loaded systems.
In particular, it should not be used to repair air conditioner radiators, but can be used to repair radiator motors.
High temperature aluminum soldering. When soldering aluminum radiators, soldering is used in factories. Its characteristic lies in the fact that the melting point of the solder is only 20-40 degrees below the melting point of the metal itself. For this soldering, as a rule, a special high-temperature paste (for example, nylon) is used, which is used for soldering and then sintered in special furnaces in a protective gas environment.
This soldering process is characterized by high strength and low corrosion resistance of the resulting joints, since the solder is used as a composition close to the base metal. Such solder is ideal for thin-walled products, but its technology is rather complicated and completely useless for repairs.
The second type of brazing aluminum is flame brazing.  Special self-tapping rods are used as solder (eg HTS 2000, Castolin 21 F, etc.).
Special self-tapping rods are used as solder (eg HTS 2000, Castolin 21 F, etc.).
Acetylene, propane and preferably a hydrogen flame (hydrolysis) are used for heating. The technology is next. First, the torch flame heats the metal, and then the soldering iron is carefully filled into the area to be soldered. When the rod melts, the flame is removed. The melting point of the rod is not much lower than the temperature of the base plate, so it must be heated carefully so that it does not come off.
It should be noted that this type of solder is very, very expensive and can be as high as $300. for 1 kilogram. As a rule, it is used for local repairs.
So which is better?
Baker melts at home: step by step video
Soldering or welding aluminum? Now we can answer this question. If the thickness of the metals is more than 0.2-0.3 mm, then use argon arc welding. In particular, argon welding of simple honeycomb balm emitters, trays, fenders, brackets, alloy wheels, steering gear, engine head, etc. The resulting weld. It is a monolithic, chemically resistant and strong bond.
If the thickness of the metals is less than 0.2-0.3 mm, it is better to use aluminum brazing. Firstly, it is used to solder thin honeycomb wall radiators from the engine, which is very difficult to drink with argon. Lower temperature soft soldering is better, if not used at all, as these seams are much less strong and less chemically resistant.
In addition, acid fluxes used in low temperature soldering can destroy both ordinary metals and solder joints in a relatively short time.
Most common metals cannot be hardened by heat treatment. However, almost all metals are hardened—to one degree or another—by forging, rolling, or bending. This is called hardening or hardening of metal.
Annealing is a type of heat treatment for softening metal that has become hard-worked - riveted so that it can continue to be cold worked.
Cold working: copper, lead and aluminum
Ordinary metals differ greatly in their degree and rate of strain hardening - work hardening or work hardening.
Copper quickly riveted as a result of cold forging, and, therefore, quickly reduces its ductility and ductility. Therefore, copper requires frequent annealing so that it can be further processed without the risk of destruction.
On the other hand, lead can be hammered into almost any shape without annealing and without the risk of breaking it.
Lead has such a margin of plasticity that allows it to obtain a large plastic deformation with a very small degree of deformation work hardening. However, although copper is harder than lead, it is generally more malleable.
Aluminum can withstand a very large amount of plastic deformation from hammer forming or cold rolling before it needs to be annealed to restore its plastic properties.
Bare aluminum is hardened much more slowly than copper, and some sheet aluminum alloys are too hard or brittle to allow much work hardening.
Cold working of iron and steel
Commercial pure iron can be cold worked to large degrees of deformation before it becomes too hard for further work.
Impurities in iron or steel impair the cold workability of the metal to the extent that most steels cannot be cold worked, except of course special low carbon steels for the automotive industry. At the same time, almost all steels can be successfully processed plastically in a red-hot state.
Why do we need annealing of metals
The precise nature of the annealing process to which the metal is subjected depends largely on the purpose of the annealed metal.
There is a significant difference in annealing methods between annealing in factories where a huge amount of sheet steel is produced, and annealing in a small car workshop, when only one part needs to be treated.
In short, cold working is plastic deformation by destroying or distorting the grain structure of the metal.
During annealing, the metal or alloy is heated to a temperature at which recrystallization occurs - the formation of new grains instead of old - deformed and elongated - grains - non-deformable and round. Then the metal is cooled at a given rate. In other words, crystals or grains within the metal that have been displaced or deformed during cold plastic working are allowed to rebuild and recover to their natural state, but at an elevated annealing temperature.
Annealing of iron and steel
Iron and low carbon steels need to be heated to about 900 degrees Celsius and then allowed to cool slowly to achieve the greatest "softness" possible.
At the same time, measures are taken to prevent contact of the metal with air in order to avoid oxidation of its surface. When this is done in a small car repair shop, warm sand is used for this.
High carbon steels require similar treatment, except that the annealing temperature for them is lower at about 800 degrees Celsius.
Copper annealing
Copper is annealed at a temperature of about 550 degrees Celsius when the copper is heated to a dark red color.
After heating, the copper is cooled in water or allowed to cool slowly in air. The cooling rate of copper after heating at the annealing temperature does not affect the degree of "softness" of this metal. Rapid cooling has the advantage of removing scale and dirt from the metal.
Aluminum annealing
Aluminum is annealed at a temperature of 350 degrees Celsius.
Heat treatment of non-ferrous alloys
In factories, this is done in suitable ovens or salt baths. In the workshop, aluminum is annealed with a gas burner. They say that at the same time, tinder is rubbed on the surface of the heated metal with a wooden torch.
When the wood starts to leave black marks, the aluminum has been annealed. Sometimes a piece of soap is used instead of wood: when the soap begins to leave brown marks, the heating must be stopped. The aluminum is then cooled in water or left to cool in air.
Zinc annealing
Zinc becomes malleable again at temperatures between 100 and 150 degrees Celsius.
This means that it can be annealed in boiling water. Zinc needs to be processed while it is hot: when it cools, it loses its malleability greatly.
Copper is widely used in the manufacture of products for various purposes: vessels, pipelines, electrical distribution devices, chemical equipment, etc. The variety of uses of copper is associated with its special physical properties.
Copper has high electrical and thermal conductivity and is resistant to corrosion. Copper density 8.93 N/cm3, melting point 1083°C, boiling point 2360°C.
Difficulties in welding copper are due to its physical and chemical properties4. Copper is prone to oxidation with the formation of refractory oxides, absorption of gases by molten metal, has a high thermal conductivity, a significant value of the coefficient of linear expansion when heated.
The tendency to oxidation necessitates the use of special fluxes during welding, which protect the molten metal from oxidation and dissolve the resulting oxides, converting them into slags.
High thermal conductivity requires the use of a more powerful flame than when welding steel. The weldability of Cu depends on its purity; the presence of B1, Pb, 3, and O3 in it especially worsens the weldability of Cu. The content of pr, depending on the grade of Cu, varies from 0.02 to 0.15%, W and Pb give copper brittleness and red brittleness. The presence of oxygen in Cu in the form of copper oxide Cu20 causes the formation of brittle metal layers and cracks that appear in the thermal zone. influence.
Copper oxide forms a fusible eutectic with copper, which has a lower melting point. The eutectics are located around the copper grains and thus weaken the bond between the grains.
The process of Cu welding is affected not only by oxygen dissolved in copper, but also by oxygen absorbed from the atmosphere. In this case, along with copper oxide CuO, copper oxide CuO is formed. When welding, both of these oxides impede the gas welding process, so they must be removed with a flux.
Hydrogen and carbon monoxide also adversely affect the Cu welding process.
As a result of their interaction with copper oxide CuO, water vapor and carbon dioxide are formed, which form pores in the weld metal. To avoid this phenomenon, copper welding must be carried out with a strictly normal flame. The purer the C and the less 0-2 it contains, the better it welds.
According to GOST 859-78, the industry for the manufacture of welded structures produces copper grades M1r, M2r M3r, which has a reduced content of Oa- (up to 0.01%).
Butt and fillet joints have been used in Cu gas welding, T-joints and lap joints do not give good results.
Before welding, the edges to be welded must be cleaned of dirt, oil, oxides and other contaminants in an area of at least 30 mm from the welding point. Welding points are cleaned manually or mechanically with steel brushes. Welding of copper up to 8 mm thick is carried out without cutting edges, and with a thickness of more than 3 mm, an X-shaped cutting of edges is required at an angle of 45 ° on each side of the joint. Dullness is made equal to 0.2 of the thickness of the metal being welded. Due to the increased fluidity of copper in the molten state, thin sheets are welded end-to-end without a gap, and sheets over 6 mm are welded on graphite and carbon linings.
The power of the welding flame when welding copper up to 4 mm thick is selected based on the consumption of acetylene 150-175 dm3 / h per 1 mm of the thickness of the welded metal, with a thickness of up to 8-10 mm, the power is increased to 175-225 dm8 / h.
For large thicknesses, welding with two torches is recommended - one is heated, and the other is welding. To reduce heat dissipation, welding is performed on an asbestos lining. To compensate for large heat losses due to removal to the heat-affected zone, preliminary and concomitant heating of the welded edges is used.
The edges are heated by one or more burners.
The flame for Cu welding is chosen strictly normal, since the oxidizing flame causes strong oxidation, and with the carburizing flame, pores and cracks appear. The flame should be soft and should be directed at a greater angle than when welding steel. Welding is carried out by a reduction zone, the distance from the end of the core to the metal to be welded is 3-6 mm.
During the welding process, the heated metal must be protected by a flame at all times. Welding is carried out in both left and right ways, however, the right way is most preferable when welding copper. Welding is carried out at maximum speed without interruption.
Welding is on the rise. The angle of inclination of the torch mouthpiece to the workpiece to be welded is 40-50°, and that of the filler wire is 30-40°. When performing vertical seams, the angle of inclination of the mouthpiece of the burner is 30 ° and welding is carried out from the bottom up. When welding copper, it is not recommended to fasten parts with tacks. Long seams are welded in a free state in a reverse step way.
Gas welding of copper is performed in only one pass.
The composition of the filler wire greatly influences the Cu gas welding process. For welding, rods and wire are used as an additive according to GOST 16130-72 of the following grades: M1, MSr1, MNZh5-1, MNZhKT5-1-0.2-0.2.
Error 503 Service Unavailable
Welding wire MSr1 contains 0.8-1.2% silver. The diameter of the filler wire is selected depending on the thickness of the metal to be welded and is taken equal to 0.5-0.75 8, where 5 is the thickness of the metal, mm, but not more than 8 mm.
The welding wire should melt calmly, without splashing. It is desirable that the melting temperature of the filler wire is lower than the melting temperature of the base metal. To protect Cu from oxidation, as well as to deoxidize and remove oxides formed into slag, welding is carried out with a flux. Fluxes are made from oxides and salts of boron and sodium. Fluxes for Cu welding are used in the form of powder, paste and in gaseous form. Fluxes No. 5 and 6 containing salts of phosphoric acid must be used when welding with wire that does not contain phosphorus and silicon deoxidizers.
Cu welding can also be performed using BM-1 gaseous flux, in this case the burner tip must be increased by one number in order to reduce the heating rate and increase the power of the welding flame. When using a gaseous flux, the KGF-2-66 installation is used. Powdered flux is sprinkled on the welding spot by 40-50 mm on both sides of the weld axis. Flux in the form of a paste is applied to the edges of the metal to be welded and to the filler rod. Flux residues are removed by washing the seam with a 2% solution of nitric or sulfuric acid.
To improve the mechanical properties of the deposited metal and increase the density and.
plasticity of the seam after welding, it is recommended to forge the seam metal. Details with a thickness of up to 4 mm are forged in a cold state, and with a greater thickness - when heated to a temperature of 550-600°C.
Additional improvement of the seam after forging gives heat treatment - heating to 550-600°C and cooling in water. The products to be welded are heated with a welding torch or in a furnace. After annealing, the weld metal becomes ductile.
⇐ Previous27282930313233343536Next ⇒
Publication date: 2015-01-26; Read: 455 | Page copyright infringement
studopedia.org - Studopedia.Org - 2014-2018. (0.001 s) ...
Home>>Non-ferrous welding>>Copper to steel welding
Welding of copper and its alloys with steel. How to weld copper and steel?
In practice, welding of copper and steel is most often carried out in butt joints. Depending on the nature of the structure, the seams in such a connection can be external and internal.
For welding brass to steel, gas welding is best, and for welding red copper to steel, metal arc welding is best.
Good results are also obtained when welding with carbon electrodes under a layer of flux and gas welding under a flux BM-1. Often in practice, gas welding of brass to steel is performed using copper as a filler material.
The preparation of welded edges with the same thickness of non-ferrous metal and steel is carried out in the same way as when welding ferrous metals.
Sheets with a thickness of less than 3 mm are welded without cutting, and sheets, starting from 3 mm, with beveled edges.
With insufficient bevel edges, or if there is dirt on the ends of the parts to be welded, it is impossible to achieve good penetration. Based on this, when welding parts of large thicknesses in which an X-shaped groove is made, blunting should not be done.
Welding copper with steel is a difficult task, but quite feasible for surfacing and welding, for example, parts of chemical equipment, copper wire with a steel block.
The quality of welding of such joints satisfies the requirements for them. The strength of copper can be increased by introducing up to 2% iron into its composition. With more iron, the strength begins to fall.
When welding with a carbon electrode, direct current of direct polarity must be used.
The voltage of the electric arc is 40-55V, and its length is approximately 14-20mm. Welding current is selected in accordance with the diameter and quality of the electrode (carbon or graphite) and is in the range of 300-550A. The flux used is the same as for welding copper, the composition of these fluxes is given on this page.
The flux is introduced into the welding zone, pouring it into the groove.
 The welding method is used "left".
The welding method is used "left".
The best results when welding copper to steel busbars are obtained when welding "in the boat". The scheme of such welding is shown in the figure. First, the copper edges are heated with a carbon electrode, and then welding is carried out with a certain position of the electrode and filler rod (see figure). Welding speed is 0.25m/h. Welding of copper with cast iron is carried out using the same technological methods.
Welding of low-alloyed bronze of small thickness (up to 1.5 mm) to steel up to 2.5 mm thick can be carried out with an overlap with a non-consumable tungsten electrode in an argon environment on an automatic machine with a filler wire with a diameter of 1.8 mm fed from the side.
In this case, it is very important to direct the arc to the overlap from the copper side. Such welding modes: current strength 190A, arc voltage 11.5V, welding speed 28.5m/h, wire feed speed 70m/h.
Copper and brass weld well to steel by flash butt welding.
With this method of welding, the steel edges are melted quite strongly, and the edges of non-ferrous metal are slightly melted. Taking into account this circumstance, and taking into account the difference in the specific resistances of these metals, take the overhang for steel equal to 3.5d, for brass 1.5d, for copper 1.0d, where d are the diameters of the welded rods.
For resistance butt welding of such rods, a stick out of 2.5d for steel, 1.0d for brass and 1.5d for copper is recommended. The specific resistance of precipitation is taken in the range of 1.0-1.5 kg/mm2.
In practice, it often becomes necessary to weld studs with a diameter of 8-12 mm from copper and its alloys to steel, or steel studs to copper products.
Such welding is carried out on a direct current of reverse polarity under a fine flux grade OSC-45 without preheating.
Copper studs up to 12 mm in diameter or L62 brass studs up to 10 mm in diameter at a current of 400A are well welded to steel or cast iron.
And studs made of brass brand LS 59-1 are not used for welding.
Steel studs are poorly welded to copper and brass products.
How is copper welding done at home?
If you put a copper ring 4 mm high on the end of the stud, up to 8 mm in diameter, then the process of welding metals proceeds satisfactorily. The same studs with a diameter of 12mm for bronze brand Br. OF 10-1 are welded well. For arc welding of copper and steel, K-100 electrodes provide the best results.
When developing a technology for the heat treatment of copper and its alloys, two of their features must be taken into account: high thermal conductivity and active interaction with gases during heating. When heating thin products and semi-finished products, thermal conductivity is of secondary importance. When massive products are heated, the high thermal conductivity of copper is the reason for their faster and more uniform heating over the entire cross section compared, for example, with titanium alloys.Due to the high thermal conductivity, hardenability problems do not arise during hardening heat treatment of copper alloys. With the dimensions of semi-finished products and products used in practice, they are calcined through.
Copper and alloys based on it actively interact with oxygen and water vapor at elevated temperatures, at least more intensively than aluminum and its alloys. In connection with this feature, protective atmospheres are often used in the heat treatment of semi-finished products and products made from copper and its alloys. , while protective atmospheres are rare in aluminum heat treatment technology.
Annealing of copper and its alloys is carried out in order to eliminate those deviations from the equilibrium structure that arose during solidification or as a result of mechanical action or previous heat treatment.
Homogenization annealing consists in heating the ingots to the maximum possible temperature, which does not cause melting of the structural components of the alloys. Segregation phenomena in copper and brass develop insignificantly, and heating of the ingots under hot pressure treatment is sufficient for their homogenization.
The main copper alloys that need homogenization annealing are tin bronzes, since the compositions of the liquid and solid phases in the Cu-Sn system are very different, and therefore intense dendritic segregation develops.
As a result of homogenization annealing, the homogeneity of the structure and chemical composition of the ingots increases. Homogenization annealing is one of the conditions for obtaining a high-quality final product.
Recrystallization annealing is one of the most common technological stages in the production of semi-finished products of copper and alloys based on it.
The onset temperature of copper recrystallization is intensively increased by Zr, Cd, Sn, Sb, Cr, while Ni, Zn, Fe, Co have little effect. The increase in the temperature of the onset of recrystallization with the simultaneous presence of several elements is non-additive, but slightly exceeds the contribution from the most effective impurity. In certain cases, for example, when lead and sulfur are introduced into copper, the total effect is higher than individual effects. Copper deoxidized by phosphorus, in contrast to oxygen-containing copper, is prone to strong grain growth during annealing. The recrystallization threshold in the presence of phosphorus shifts to higher temperatures.
The critical degree of deformation for oxygen-free copper with a grain size of the order of 2*10 in-2 cm after annealing at 800°C for 6 hours is approximately 1%. Impurities, such as iron, increase the critical degree of deformation, which for brass is 5-12% (Fig. 44).
The recrystallization temperature of brasses is also affected by the previous processing, primarily the degree of cold deformation and the grain size formed during this processing. So, for example, the time before the start of recrystallization of brass L95 at temperatures of 440 ° C is 30 minutes at a degree of cold deformation of 30% and 1 minute at a degree of deformation of 80%.
The size of the initial grain affects the crystallization process opposite to the increase in the degree of deformation. For example, in the L95 alloy with an initial grain size of 30 and 15 μm, annealing after 50% deformation at a temperature of 440°C leads to recrystallization after 5 and 1 min, respectively. At the same time, the initial grain size does not affect the recrystallization rate if the annealing temperature exceeds 140°C.
On fig. 45 shows data on the effect of the composition of α-brass on the annealing temperature (degree of deformation 45% annealing time 30 min), which provides a given grain size. Under the same deformation and annealing conditions, with an increase in the zinc content, the grain size decreases, reaches a minimum, and then grows. So, for example, after annealing at 500°C for 30 min, the grain size is: in copper 0.025 mm; in brass with 15% Zn 0.015 mm, and in brass with 35% Zn 0.035 mm. Figure 45 also shows that in α-brass grain begins to grow at relatively low temperatures and grows up to solidus temperatures. In two-phase (α + β)- and special brass, grain growth, as a rule, occurs only at temperatures at which one β-phase. For example, for brass L59, a significant grain increase begins upon annealing above a temperature of 750 ° C.
The annealing temperature of brass is chosen approximately 250–350°C higher than the temperature at which recrystallization begins (Table 16).
During annealing of copper alloys containing 32-39% Zn at temperatures above the α⇔α+β-transition, the β-phase is precipitated, which causes uneven grain growth. It is desirable to anneal such alloys at temperatures not exceeding the α⇔α+β-equilibrium line of the Cu-Zn system. In this regard, brass, which lies in composition near the point of maximum solubility of zinc in copper, should be annealed in furnaces with high temperature control accuracy and high uniformity of its distribution over the volume of copper.
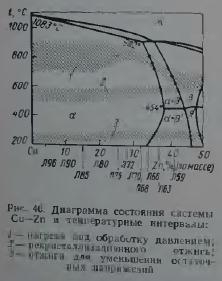
On fig. 46 shows the optimal annealing modes for simple brasses based on the results of summarizing technological recommendations accumulated in domestic and world practice. There is a tendency to increase the temperature of complete annealing of brass with an increase in the content of zinc in them.
When choosing the modes of recrystallization annealing of brasses, it should be taken into account that alloys lying near the α / α + β phase boundary (Fig. 46), due to the variable solubility of zinc in copper, can be thermally strengthened. Quenching of brasses containing more than 34% Zn makes them prone to aging (Fig. 47), and the ability to harden during aging increases with increasing zinc content up to 42%. This type of thermal hardening of brass has not found practical application. Nevertheless, the cooling rate of L63 type brass after recrystallization annealing affects their mechanical properties. The possibility of decomposition of supersaturated solutions in α-brass containing more than 34% Zn, and in α+β-brass, should also be taken into account when choosing annealing regimes to reduce stresses. Strong cold deformation can accelerate the decomposition of supersaturated α- and β-solutions during annealing.
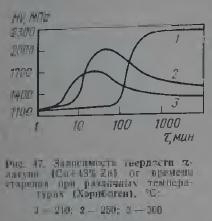
According to the literature, the temperature of the onset of recrystallization of brass L63 ranges from 250 to 480 ° C. The finest grained structure in the L63 alloy is formed after annealing at temperatures of 300-400 ° C. The higher the degree of previous cold deformation, the smaller the size of the recrystallized grain and the greater the hardness (Fig. 48) under the same annealing conditions.
The quality of the annealed material is determined not only by its mechanical properties, but also by the size of the recrystallized grain. The grain size in a fully recrystallized structure is fairly uniform. Under incorrectly set modes of recrystallization annealing, two groups of grains of different sizes are clearly found in the structure. This so-called double structure is particularly undesirable in deep drawing, bending or polishing and etching operations on the workpiece.
With an increase in grain size to a certain limit, the formability of brass improves, but the surface quality deteriorates. On the surface of the product with a grain size of more than 40 microns, a characteristic roughness "orange peel" is observed.
The stages of the evolution of the deformed structure are significantly extended in time, and therefore it seems possible to obtain a partially or completely recrystallized structure with fine grains by varying the annealing time. Semi-finished products with an incompletely recrystallized structure with a very small grain size are stamped without the formation of an "orange peel".
Incomplete annealing, the duration of which is determined by the degree of preliminary deformation, is carried out in the range of 250-400 ° C. To comply with the exact technological regime, such annealing should be carried out in broaching furnaces, where the operating temperature and holding time (drawing speed) are strictly controlled.
Incomplete annealing is mainly used to reduce residual stresses, which can lead to so-called "seasonal cracking". This type of corrosion, inherent in brass with a content of more than 15% Zn, consists in the gradual development of intergranular cracks under the simultaneous action of stress (residual and applied) and specific chemical reagents (for example, solutions and vapors of ammonia, solutions of mercury salts, wet sulfuric anhydride, various amines etc.). It is believed that the sensitivity of brasses to seasonal cracking is due more to the inhomogeneity of stress than to their absolute value.
The effectiveness of the annealing to reduce the residual stresses is checked by the mercury sample test. The mercury breakdown test method gives a qualitative assessment of the presence of residual stresses. It is based on the different behavior of stressed and unstressed material when exposed to mercury nitrate. During the test, longitudinal and transverse cracks appear on the stressed material, visible to the naked eye. They appear in places of tensile stresses, which can cause the destruction of the product in operation or during storage as a result of corrosion cracking.
Brass annealing modes to reduce residual stresses are given in fig. 46 and in table. 16.
Parshev 01-09-2005 02:01
"The temperature can be determined quite accurately using a small piece of copper foil (the size of a match head), which is placed on the surface of the heated part. At a temperature of 400 ° C, a greenish flame appears above the foil.
Hardening of a preheated copper part occurs during slow cooling in air. For annealing, the heated part is rapidly cooled in water. During annealing, copper is heated to red heat (600 ° C), while hardening - up to 400 ° C, determining the temperature also with a piece of copper foil.
In order for brass to become soft, easily bent, forged and well drawn, it is annealed by heating to 500 ° C and slow cooling in air at room temperature.
Interestingly, the annealing of copper and brass occurs in the opposite way - there with rapid cooling, there with slow cooling.
When forming sleeves, it is recommended to anneal after 2 operations.
Remus 02-09-2005 01:49
After what 2 surgeries?
Parshev 02-09-2005 02:11
Sleeve molding operations. For example, re-crimping to a different size is done by running through the dies.
ABAZ 05-09-2005 08:12
sorry,translit zaklinilo.
Anyman 06-09-2005 08:27
capercaillie 11-09-2005 15:13
Take a gas-foam brick, drill holes in it for your caliber, one-third of the product deep, insert a workpiece into the holes with the bottom up, and heat the product with a gas burner or hairdryer until it glows slightly and drop the product into water or cool to room temperature in a conductor (brick).
TSV 11-09-2005 22:29
And if you just stuff the sleeves into the holder, put the holder in a bath of water, which should be poured below the slope, and heat the protruding muzzles with a burner?
Sleeves naturally without capsules, so that water flows inside.
The muzzle will be annealed, and the rest will remain intact
And no bricks to drill
Machete 12-09-2005 12:54
The couple will be like in a bath.
capercaillie 12-09-2005 13:18
Try. Tell us.
TSV 12-09-2005 20:34
Nothing. There is no burner. Don't heat it up with a hair dryer.
Tried on a regular gas burner. He wrapped it with a wet rag, and into the fire. Seems to be OK. Only the fire is weak.
TSV 12-09-2005 23:34
The couple will be like in a bath.
There shouldn't be a couple. Now, if I had heated it up and lowered it, then yes, I would have received a steam room.
But after all, in this case, everything would be heated, and not just one muzzle.
Machete 13-09-2005 12:23
When you say "should" - knock on wood (Mayan proverb).
TSV 13-09-2005 12:29
quote: Originally posted by Machete:
When you say "should" - knock on wood (Mayan proverb).
Then let's just say - it was not when I kept it on gas in a wet rag.
If annealed in a good way, then it is necessary that the sleeve rotates around the axis. Otherwise, the side heats up, and the rest remains unheated. It can be seen from the trace of runaway.
Machete 13-09-2005 02:02
I like some version of Gennady Mikhailych more. Although our interest is purely gastronomic - yet.
TSV 13-09-2005 21:10
Like drilling holes in bricks?
I don’t know what that brick is, but the metal needs to be cooled, except for the place of heating.
capercaillie 13-09-2005 21:56
Sergey, and according to technology, you write to the manufacturer of bullets.
And the brick is cut with a knife.
Machete 13-09-2005 22:05
With water, while heating the muzzle, you can’t cool the sleeve very well - it’s brass, the thermal conductivity is hurt.
TSV 13-09-2005 22:45
quote: Originally posted by Machete:
With water, while heating the muzzle, you can’t cool the sleeve very well - it’s brass, the thermal conductivity is hurt.
I won’t be able to try it tomorrow (running around on business), then I’ll test brass in water.
Although the metal is thermally conductive, it cannot be heated below the water level. After all, we are only interested in the annealed muzzle.
Machete 14-09-2005 01:13
quote: Originally posted by TSV:Although the metal is thermally conductive, it cannot be heated below the water level.
Didn't quite screw up. What is meant?
TSV 14-09-2005 01:28
If the sleeve is stuffed into something porous, then there will be a weak heat sink. And heating the muzzle at the same time will heat up the rest. Up to half, the sleeve should definitely warm up and turn black, or even warm up more.
Water takes away heat, and the part that is farthest from the water warms up more.
The last time I wrapped the cartridge case in a rag and wet it so that the water drained. Then he threw it into the fire. A wet rag did not allow the body of the sleeve to heat up. The muzzle and slope warmed up.
Next time I'll try heating the sleeve sticking out of the water. I will write about the result. Now there is no gas burner at hand
Machete 14-09-2005 01:39
So this running water is needed, according to the type of cooling of the coil in the moonshine still, otherwise there will be no kin.
TSV 15-09-2005 20:22
In general, I checked the version.
Basically it works. But the power of a gas soldering iron is not enough for heating, as the water takes heat. But the sleeve is not annealed below the water. There is no hiss or sizzle. Not the temperature to instantly heat all the water.
Tried without water, empty. It warmed up quickly, but due to the heat transfer, half of the sleeve managed to warm up.
If the view does not bother that it is below the slope, then it will go without water. But you still need to rotate. Otherwise, on the one hand, the stain burns out, and on the other hand, the heating is weaker.
Parshev 16-09-2005 17:05
2 ParshevWhere did the information come from? The writing style is not similar to technical literature, closer to the household
You checkers or go?
Anyman 20-09-2005 08:27
quote: Originally posted by Parshev:You checkers or go?
Technical literature describes how to do it in a factory or laboratory environment, do you have them?
Anyman 20-09-2005 08:54
quote: Originally posted by capercaillie:
Bullet manufacturers recommend:
Take a gas-foam brick, drill holes in it for your caliber, one-third of the product deep, insert a workpiece into the holes with the bottom up, and heat the product with a gas burner or hairdryer until it glows slightly and drop the product into water or cool to room temperature in a conductor (brick).
2 capercaillie
Do you mean ordinary building bricks or something special like fireclay?
capercaillie 20-09-2005 10:12
Yes, at every construction fair they sell
a gas-foam brick bought a block and sawed himself any kind of bricks.
For annealing I use a gas burner.
Identity sell, refueled from cans for lighters.
RAY 27-09-2005 15:20
quote: Originally posted by Anyman:On the one hand, you are right. But remembering from the training days that heat treatment is not the easiest thing, I would definitely consult a thermist or look in the appropriate reference book. After all, if everything can be more or less unambiguous with copper, then brass can be very different in chemical composition and, accordingly, suitability for heat treatment.
For example annealing temperature for brass:Brass L96: 540 - 600 degrees;
Brass L90 - L62: 600 - 700 degrees;Since people who are counting every powder have gathered here, everything should be accurate
-----------
Uh-huh ... they dragged me a lot of shells for analysis - more and more L63 was ...
L96 and L90 - even in color - COPPER ... more and more L63 and L65 seemed to always go to the sleeves ...
Anyman 27-09-2005 20:00
Duc, in L96 copper 95-97% is therefore copper in color. In L63 62-65%
tov_Mauser 14-10-2005 11:04
ingredients: Nagan revolver shells
tools: pliers, rag, gas burner on the stove
we wet the rag and wring it out, wrap the handles of the pliers, with the pliers we take the sleeve behind the ..pu and at an angle of 45 we heat it in a flame (better at dusk - so that the glow of the metal can be seen) we heat the neck to a dull redness, after which we put the sleeve aside to cool down. When heated, massive pliers remove heat from the base of the sleeve - which is clearly seen as the metal warms up
the output is high-quality cartridge cases that do not crack during repeated reloading and rolling / flaring gun
HEAT TREATMENT OF COPPER AND BRASS
Copper.
Copper is used for the production of sheets, tape, wire by cold deformation. In the process of deformation, it loses plasticity and acquires elasticity. The loss of plasticity makes hardening, broaching and drawing difficult, and in some cases makes it impossible to further process the metal.
To remove hardening or work hardening and restore the plastic properties of copper, recrystallization annealing is carried out according to the regime: heating to a temperature of 450–500 ° C at a rate of 200–220 ° C / h, exposure, depending on the configuration and weight of the product, from 0.5 to 1 .5 h, cooling in still air. The structure of the metal after annealing consists of equiaxed crystals, strength σv = 190 MPa, relative elongation δ = 22%.
Brass.
An alloy of copper and zinc is called brass. There are two-component (simple) brasses, consisting only of copper, zinc and some impurities, and multi-component (special) brasses, into which one or more alloying elements (lead, silicon, tin) are introduced to give the alloy certain properties.
Two-component brasses, depending on the method of processing, are divided into wrought and foundry.
wrought two-component brasses (L96, L90, L80, L63, etc.) have high ductility and are well processed by pressure; they are used for the manufacture of sheets, tapes, strips, pipes, wires and bars of various profiles.
Foundry brass is used for casting shaped parts. In the process of cold working by pressure, two-component brasses, like copper, receive work hardening, as a result of which strength increases and ductility decreases. Therefore, such brass is subjected to heat treatment - recrystallization annealing according to the regime: heating to 450-650 ° C, at a speed of 180-200 ° C / h, exposure 1.5-2.0 h and cooling in still air. Brass strength after annealing σ Β = 240-320 MPa, relative elongation δ = 49-52%
Brass products with high internal stress in the metal are prone to cracking. During long-term storage in air, longitudinal and transverse cracks form on them. To avoid this, products are subjected to low-temperature annealing at 250-300 ° C before long-term storage.
Availability in multicomponent(special)latunyah alloying elements (manganese, tin, nickel, lead and silicon) gives them increased strength, hardness and high corrosion resistance in atmospheric conditions and sea water. Brass alloyed with tin, for example, LO70-1, LA77-2 and LAN59-3-2, called marine brass, have the highest stability in sea water; they are used mainly for the manufacture of parts for marine vessels.
According to the processing method, special brasses are divided into wrought and foundry. Wrought brass is used to produce semi-finished products (sheets, pipes, tapes), springs, clock and instrument parts. Cast multicomponent brasses are used for the manufacture of semi-finished products and shaped parts by casting (propellers, blades, fittings, etc.). The required mechanical properties of special brass are provided by their heat treatment, the modes of which are given in the table. To obtain fine grains, before deep drawing, deformable brasses for sheets, strips, and strips are subjected to annealing at a temperature of 450-500°C.
Heat Treatment Modes for Special Brass *
|
Alloy grade |
Purpose of processing |
Type of processing |
Heating temperature, °C |
Exposure, h |
|
Wrought brass |
||||
|
Hardening removal |
Recrystallization annealing |
|||
|
stress relief |
low annealing |
|||
|
Cast brass |
||||
|
stress relief |
Recrystallization annealing |
|||
* Cooling medium - air.
THERMAL STRENGTHENING OF BRONZE
Bronze is an alloy of copper with tin, lead, silicon, aluminium, beryllium and other elements. According to the main alloying element, bronzes are divided into tin and tinless (special), according to mechanical properties - into wrought and foundry.
Deformable pewter bronze grades Br.OF8-0.3, Br.OTs4-3, Br.OTsS4-4-2.5 are produced in the form of rods, tapes, wire for springs. The structure of these bronzes consists of an α-solid solution. The main type of heat treatment of bronzes is high annealing according to the regime: heating to 600-650 ° C, holding at this temperature for 1-2 hours and rapid cooling. Strength after annealing σ in - 350-450 MPa, relative elongation b = 18-22%, hardness HB 70-90.
Foundry pewter bronze grades Br.OTs5-5-5, Br.OSNZ-7-5-1, Br.OTsSZ,5-7-5 are used for the manufacture of anti-friction parts (bushings, bearings, liners, etc.). Cast tin bronzes are annealed at 540-550°C for 60-90 minutes.
Tinless bronze Br.5, Br.7, Br.AMts9-2, Br.KN1-3 and other brands have high strength, good anticorrosion and antifriction properties. Gears, bushings, membranes and other parts are made from these bronzes. To facilitate the pressure treatment, the bronzes are subjected to homogenization at 700-750°C, followed by rapid cooling. Castings with internal stresses are annealed at 550°C with a holding time of 90-120 minutes.
Most commonly used in industry double - aluminum bronze grades Br.A5, Br.A7 and bronze, additionally alloyed with nickel, manganese, iron and other elements, for example Br.AZhN10-4-4. These bronzes are used for a variety of bushings, flanges, seat guides, gears, and other small, heavily loaded parts.
Double aluminum bronzes are subjected to hardening and tempering according to the regime: heating for hardening up to 880-900 ° C at a speed of 180-200 ° C / h, holding at this temperature for 1.5-2 hours, cooling in water; tempering at 400-450°C for 90-120 minutes. The structure of the alloy after quenching consists of martensite, after tempering, from a fine mechanical mixture; bronze strength σ in = 550 MPa, δ = 5%, hardness HB 380-400.
beryllium bronze Br.B2 is an alloy of copper with beryllium. Unique properties - high strength and elasticity with simultaneous chemical resistance, non-magnetism and the ability to heat harden - all this makes beryllium bronze an indispensable material for the manufacture of clock and instrument springs, membranes, springy contacts and other parts. High hardness and non-magnetism make it possible to use bronze as a percussion instrument (hammers, chisels) that does not form sparks when hitting stone and metal. Such a tool is used when working in explosive environments. Bronze Br.B2 is hardened at 800-820°C with cooling in water, and then subjected to artificial aging at 300-350°C. In this case, the strength of the alloy σ Β = 1300 MPa, hardness HRC37-40.
THERMAL STRENGTHENING OF ALUMINUM ALLOYS
Deformable aluminum alloys divided into non-hardened by heat treatment and hardened. To hardened aluminum alloys include alloys of the grade AMts2, AMg2, AMgZ, which have low strength and high ductility; they are used for products obtained by deep drawing, hardened by cold pressure treatment (hardening).
The most common alloys hardened heat treatment. These include duralumin grades D1, D16, D3P, which include aluminum, copper, magnesium and manganese. The main types of thermal hardening of duralumin are hardening and aging. Hardening is carried out at 505-515 ° C, followed by cooling in cold water. Aging is used both natural and artificial. With natural aging, the alloy is kept for 4-5 days, with artificial aging - 0.8-2.0 hours; aging temperature - not lower than 100-150°C; strength after processing σ Β = 490 MPa, 6=14%. Alloys D1 and D16 are used for the manufacture of parts and elements of building structures, as well as products for aircraft.
Avial (AB, AVT, AVT1) is a deformable alloy with higher ductility, weldability and corrosion resistance than duralumin; subjected to quenching in water at 515-525 ° C and aging: alloys AB and AVT - natural, alloy AVT1 - artificial at 160 ° C with an exposure of 12-18 hours. Aviation is used for the production of sheets, pipes, blades of helicopter propellers etc.
High-strength (σ in = 550-700 MPa) aluminum alloys B95 and B96 have lower ductility than duralumin. The heat treatment of these alloys consists in quenching at 465–475 ° C with cooling in cold or hot water and artificial aging at 135–145 ° C for 14–16 hours. Alloys are used in aircraft construction for loaded structures that operate for a long time. time at 100-200°C.
Forging aluminum alloys grades AK1, AK6, AK8 are subjected to hardening at 500-575 ° C with cooling in running water and artificial aging at 150-165 ° C with an exposure of 6-15 hours; alloy strength σ Β = 380-460 MPa, relative elongation δ = 7-10%.
Foundry aluminum alloys called silumi-nami. The most common heat-hardenable alloys are AL4, AL6, and AL20. Castings from AL4 and AL6 alloys are quenched at 535–545°C with cooling in hot (60–80°C) water and subjected to artificial aging at 175°C for 2– 3 h; after heat treatment σ in = 260 MPa, δ = 4-6%, hardness HB 75-80. To relieve internal stresses, castings from these alloys are annealed at 300°C for 5–10 h with cooling in air. Heat-resistant alloys of grades AL 11 and AL20, which are used for the manufacture of pistons, cylinder heads, boiler furnaces, operating at 200–300 ° C, are subjected to hardening (heating to 535–545 ° C, holding at this temperature for 3–6 h and cooling in running water), as well as stabilizing vacation at 175-180 ° C for 5-10 hours; after heat treatment σ in =300-350 MPa, δ=3-5%.
HEAT TREATMENT OF MAGNESIUM AND TITANIUM ALLOYS
magnesium alloys.
The main elements in magnesium alloys (except magnesium) are aluminum, zinc, manganese and zirconium. Magnesium alloys are divided into wrought and cast.
Deformable magnesium alloys grades MA1, MA8, MA14 are subjected to thermal hardening according to the regime: heating for hardening up to 410-415 ° C, holding for 15-18 hours, cooling in air and artificial aging at 175 ° C for 15-16 hours; after heat treatment σ Β = 320~430 MPa, δ = 6-14%. Alloys MA2, MAZ and MA5 are not subjected to heat treatment; they are used for the manufacture of sheets, plates, profiles and forgings.
Chemical composition foundries magnesium alloys (ML4, ML5, ML12, etc.) is close to the composition of deformable alloys, but the ductility and strength of cast alloys are much lower. This is due to the rough casting structure of the alloys. Heat treatment of castings with subsequent aging promotes the dissolution of excess phases concentrated along the grain boundaries and an increase in the plasticity and strength of the alloy.
A feature of magnesium alloys is the low rate of diffusion processes (phase transformations proceed slowly), which requires a long exposure for quenching and aging. For this reason, alloys can only be quenched in air. Aging of cast magnesium alloys is carried out at 200-300°C; for hardening they are heated to 380-420 ° C; after hardening and aging σ in = 250-270 MPa.
Magnesium alloys can be used as heat-resistant, capable of operating at temperatures up to 400 ° C. Due to the high specific strength, magnesium alloys are widely used in aviation, rocket science, the automotive and electrical industries. A major disadvantage of magnesium alloys is their low resistance to corrosion in a humid atmosphere.
titanium alloys.
Titanium is one of the most important modern structural materials; has high strength, high melting point (1665° C), low density (4500 kg/m3) and high corrosion resistance even in sea water. On the basis of titanium, alloys of increased strength are formed, which are widely used in aviation and rocketry, power engineering, shipbuilding, the chemical industry and other industries. The main additives in titanium alloys are aluminum, molybdenum, vanadium, manganese, chromium, tin and iron.
Titanium alloys of grades VT5, VT6-S, VT9 and VT16 are subjected to annealing, hardening and aging. Semi-finished products (rods, forgings, pipes) from an alloy additionally alloyed with tin (VT5-1) undergo recrystallization annealing at 700–800°C in order to remove hardening. Sheet titanium alloys are annealed at 600-650°C. The duration of annealing for forgings, rods and pipes is 25-30 minutes, for sheets - 50-70 minutes.
Highly loaded parts made of VT14 alloy, operating at a temperature of 400 ° C, are hardened with subsequent aging according to the regime: hardening temperature 820-840 ° C, cooling in water, aging at 480-500 ° C for 12-16 hours; after hardening and aging: σ in = 1150-1400 MPa, 6 = 6-10%, hardness HRC56-60.